Abstract
BACKGROUND
GVHD and non-relapse mortality (NRM) remain frequent complications of HLA-matched HSCT despite the use of standard immunosuppression like tacrolimus and methotrexate. Alternative GVHD prophylaxis (PPX) strategies like T-cell depletion and post-transplant cyclophosphamide negatively impact relapse, infection, and organ toxicity, and no strategy has yet demonstrated a clear benefit for GVHD-free survival. Orca-T is an investigational cellular product comprising stem and immune cells that leverages highly purified donor regulatory T cells to control alloreactive immune responses. Unlike point-of-care graft engineering approaches, Orca-T is produced in a central GMP laboratory and has been successfully distributed to multiple centers across in the U.S. Early clinical trials using Orca-T showed a good safety profile, promising GVHD control, and potentially improved immune reconstitution. Here, we present trial results from both a single-institution Phase 1/2 trial that has completed enrollment and an ongoing multicenter Phase 1b trial.
METHODS
As of 28 July 2021, 113 patients aged 18-72 have received Orca-T for AML, ALL, MDS, lymphoma, or myelofibrosis. We present here data from 80 patients that have ≥90 days follow-up. 28 and 52 patients, respectively, received Orca-T followed by single-agent GVHD prophylaxis on a single-center Phase 2 study (NCT01660607) and a multicenter Phase Ib (NCT04013685). Orca-T products were derived from HLA-matched related (n=46) or unrelated (n=34) donors. Patients received a variety of myeloablative conditioning regimens (e.g., non-TBI, n=66; TBI-based, n=14) followed by single-agent PPX with either tacrolimus (n=73) or sirolimus (n=7). Median follow-up for these patients is 541 days (single-center) and 248 days (multicenter).
We identified a contemporaneous SOC cohort, and we reported on their clinical outcomes at Stanford (n=95) with both matched related (n=52) and unrelated (n=43) transplant recipients who received unmanipulated PBSC products (median f/u 546) and methotrexate plus tacrolimus prophylaxis.
RESULTS
The Orca-T investigational cell therapy was manufactured reliably, delivered in less than 72 hours for all patients, and every patient enrolled received Orca-T. The Treg drug product was characterized by high Treg purity of 93.8% +/- 3.1% and a dose of 2.6 +/- 0.4 x 106 per kg (equivalent between trials). An Orca-T product was produced and infused for all patients, and there were no logistics failures or infusion reactions. All patients engrafted and Orca-T patients showed earlier neutrophil (median of 12 days vs. 14 days, p<0.0001 by Mann-Whitney U) and platelet engraftment (14 vs 17 days, p<0.0001) compared to SOC.
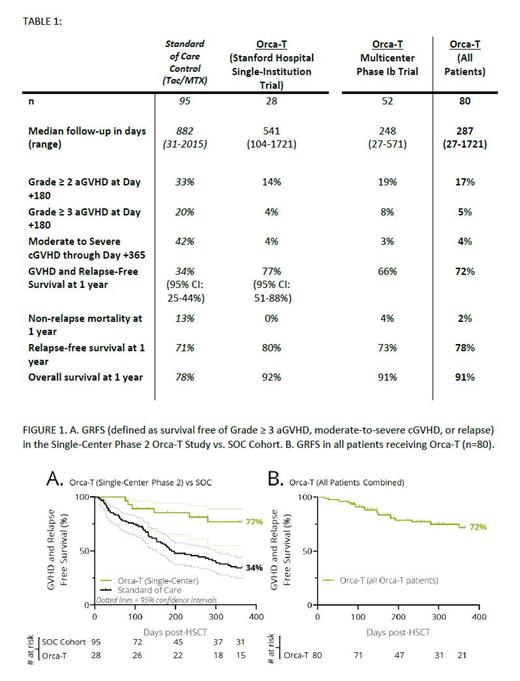
On the single-center, Phase 2 clinical trial study at Stanford there is evidence of improved 1-year GVHD and relapse-free survival (GRFS) which was 77% (CI 51-88%) for Orca-T patients vs 34% (CI 25-44%) with SOC (Figure 1A). We observed improved rates of >grade 2 acute GVHD at Day +180 (aGVHD, 14% versus 33%), moderate-to-severe chronic GVHD at 1 year (4% versus 42%) and NRM at 1 year (0% versus 13%). Relapse-free (RFS) and overall survival (OS) trended upwards for Orca-T. Severe infectious complications were rare.
Key clinical results from both Orca-T trials are summarized in Table 1; 23 of 80 patients had ≥1 year follow-up. Consistent with findings from the single-institution study, on the multicenter study, rates of moderate-to-severe cGVHD and non-relapse mortality were low at 1-year post-transplant at 3% and 4%, respectively.
For all patients who received Orca-T across both studies, we observed GRFS of 72% (Figure 1B), RFS of 78%, and OS of 91% at 1 year. These survival rates compare favorably to the contemporaneous SOC control (33%, 71% and 78%, respectively).
Immune reconstitution in Orca-T patients with single agent tacrolimus appears similar to SOC except for observable differences in the IL-2 pathway.
CONCLUSIONS
Manufacture of high precision Orca-T investigational cell therapy drug products was scaled in a central GMP with reliable distribution to centers. Patients that received Orca-T and single-agent PPX showed significantly reduced aGVHD, cGVHD and NRM. Orca-T shows promise to improve GRFS and other transplant outcomes. Orca-T has been granted Regenerative Medicine Advanced Therapy status by the FDA, and a phase 3 prospectively, randomized study is planned.
Gandhi: Gamida Cell: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDx Inc: Honoraria. Muffly: Pfizer, Amgen, Jazz, Medexus, Pfizer: Consultancy; Astellas, Jasper, Adaptive, Baxalta: Research Funding; Adaptive: Honoraria, Other: fees for non-CME/CE services: , Research Funding. Shiraz: Kite Pharma-Gilead: Research Funding. Mehta: Kadmon: Research Funding; Incyte: Research Funding; CSLBehring: Research Funding; Syndax: Research Funding. McGuirk: Allovir: Consultancy, Honoraria, Research Funding; Novartis: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Gamida Cell: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; EcoR1 Capital: Consultancy; Bellicum Pharmaceuticals: Research Funding; Pluristem Therapeutics: Research Funding; Fresenius Biotech: Research Funding; Astelllas Pharma: Research Funding; Novartis: Research Funding. Waller: Verastem Oncology: Consultancy, Research Funding; Cambium Oncology: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Arai: Magenta Therapeutics: Research Funding. Rezvani: US Department of Justice: Consultancy; Nohla Therapeutics: Other: One-time scientific advisory board; Pharmacyclics-Abbvie: Research Funding; Kaleido: Other: One-time scientific advisory board. Weng: Kite Pharma: Research Funding. Miklos: Adaptive Biotechnologies, Novartis, Juno/Celgene-BMS, Kite, a Gilead Company, Pharmacyclics-AbbVie, Janssen, Pharmacyclics, AlloGene, Precision Bioscience, Miltenyi Biotech, Adicet, Takeda: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics, Amgen, Kite, a Gilead Company, Novartis, Roche, Genentech, Becton Dickinson, Isoplexis, Miltenyi, Juno-Celgene-Bristol Myers Squibb, Allogene, Precision Biosciences, Adicet, Adaptive Biotechnologies: Research Funding; Kite, a Gilead Company, Amgen, Atara, Wugen, Celgene, Novartis, Juno-Celgene-Bristol Myers Squibb, Allogene, Precision Bioscience, Adicet, Pharmacyclics, Janssen, Takeda, Adaptive Biotechnologies and Miltenyi Biotechnologies: Consultancy; Pharmacyclics: Patents & Royalties. Frank: Kite-Gilead: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Research Funding; Allogene Therapeutics: Research Funding. Fernhoff: Orca Bio: Current Employment. Putnam: Orca Bio: Current Employment. McClellan: Orca Bio: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Shaw: Orca bio: Consultancy; mallinkrodt: Other: payments. Abedi: Seattle Genetics: Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Speakers Bureau. Meyer: Triursus Therapeutics: Current holder of stock options in a privately-held company; GigaImmune: Current holder of stock options in a privately-held company; Orca Biosystems: Research Funding; Indee, Jura: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal